orbital diagram ca
To write the orbital diagram for the Calcium atom Ca first we need to write the electron configuration for just Ca. What is the orbital diagram for Calcium Ca.
Solved A How Many Atoms Are In 35 8 Grams Of Ca Oh 2 Select One O A 2 91 X 1025 Atoms O B 2 43 Atoms O C 0 483 Atoms D 146 X 1024 Atoms Which Course Hero
The orbital diagram is drawn by using.

. 1s22s22p63s23p64s2 or Ar 4s2. 13 orbital diagram for ca Saturday October 22 2022 The. Write the full orbital diagram for each element.
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. View the full answer. An orbital diagram or orbital filling diagram is a type of notation that illustrates an atoms electron distribution and electron spin within orbitals.
Draw the partial valence-level orbital diagram Write the corresponding electron configuration for Create the atomic orbital diagram for nitrogen. Part A Enter an orbital diagram for S. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital.
An orbital diagram calculator is an online tool to get the orbital diagram of an atom. 83 6 ratings Calcium has atomic number of 20 so it has total of 20 electrons which should. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need.
Fill the orbitals starting on the left and moving toward the right even if they are Reset Help. This is called quantum jump. Atoms can jump from one orbital to another orbital in an excited state.
The orbitals are 1s 2s 2p 3s 3p and 4s. Use the Pauli exclusion principle and Hunds rule to work out. Orbital diagrams are like the configuration notation just introduced except with the spins of electrons indicated.
The ground-state electron configuration of cadmium is 1s 2 2s 2 2p 6. To do that we need to find the number o. The head-to-head overlap giving σ molecular orbitals results in greater overlap making its bonding molecular orbital the most stable and lowest energy while the σ antibonding is least.
Calcium Ca Number of electrons per shell 2 8 8 2 Number of valence electrons. Draw the orbital diagram for ion Ca 2. Therefore the magnesium full electron configuration will be 1s 2 2s 2 2p 6 3s 2.
The orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an. Part B Enter an orbital diagram. Orbital Diagram of All Elements Diagrams.
The orbital diagram for Calcium is drawn with 6 orbitals. 1s 2 2s 2 2p 6 3s 2 3p 3. So the next six electrons enter the 2p orbital and the remaining two electrons enter the 3s-orbital.
Drag the appropriate labels to their respective targets. Orbital Diagram for CalciumCa Electron configuration of calcium in the excited state. Fill the orbitals starting on the left and moving.
How to Draw Orbital Diagrams.

Draw Orbit Structure Diagram Of Calcium Oxide Cao Chemistry Shaalaa Com

Calcium Orbital Diagram Electron Configuration And Valence Electrons

Atomic Orbital Diagram Of Calcium 2 Ion Youtube
Draw The Orbital And Lewis Dot Structure Of Cao Sarthaks Econnect Largest Online Education Community

Orbital Configurations Ck 12 Foundation

Orbital Diagrams Ppt Download
Chem 2303 Supplementary Problems

How To Draw Orbital Structure Of Calcium Oxide Nitrogen And Also Of Methane Chemistry Atomic Structure And Chemical Bonding 14190737 Meritnation Com
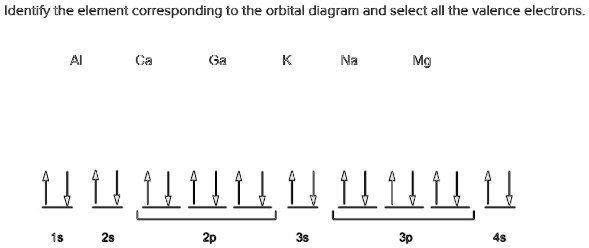
Solved Identify The Element Corresponding To The Orbital Diagram And Select All The Valence Electrons Na Mg Ld4llullu

High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World

Draw The Orbital Diagram Ca Ion And State The Number Of Three Fundamental Particles Present In It
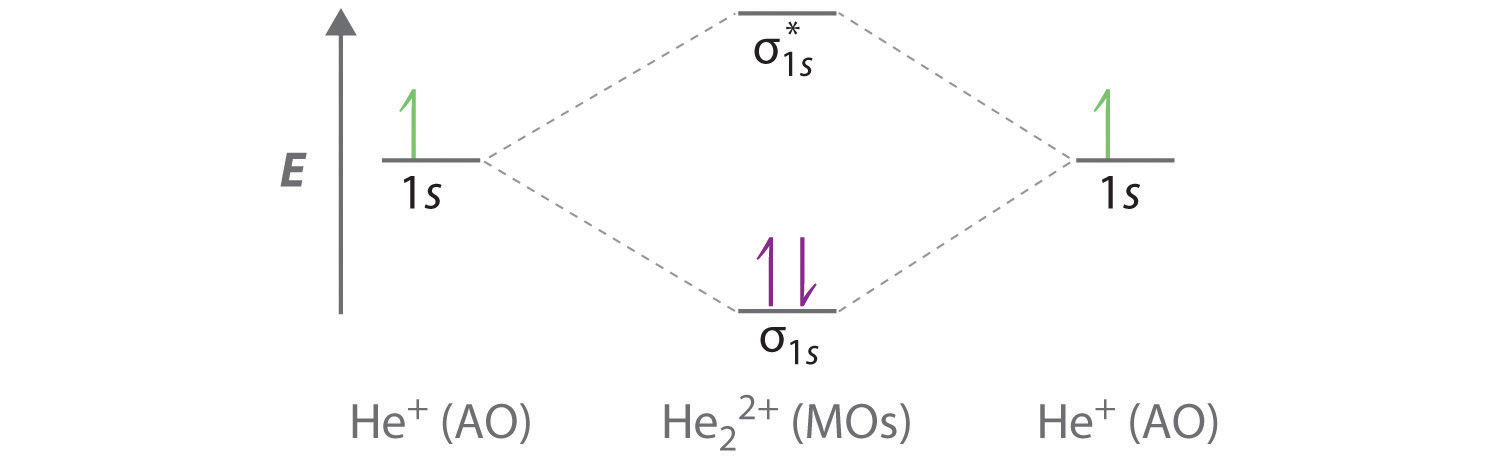
Delocalized Bonding And Molecular Orbitals

Draw An Orbital Diagram And Lewis Structure For A Li And S Homework Study Com

File Ca As Comparison Svg Wikimedia Commons

What Is The Orbital Diagram Of Calcium Ca 20 Brainly Ph

1 5 Electronic Structure Of Atoms Electron Configurations Inorganic Chemistry For Chemical Engineers
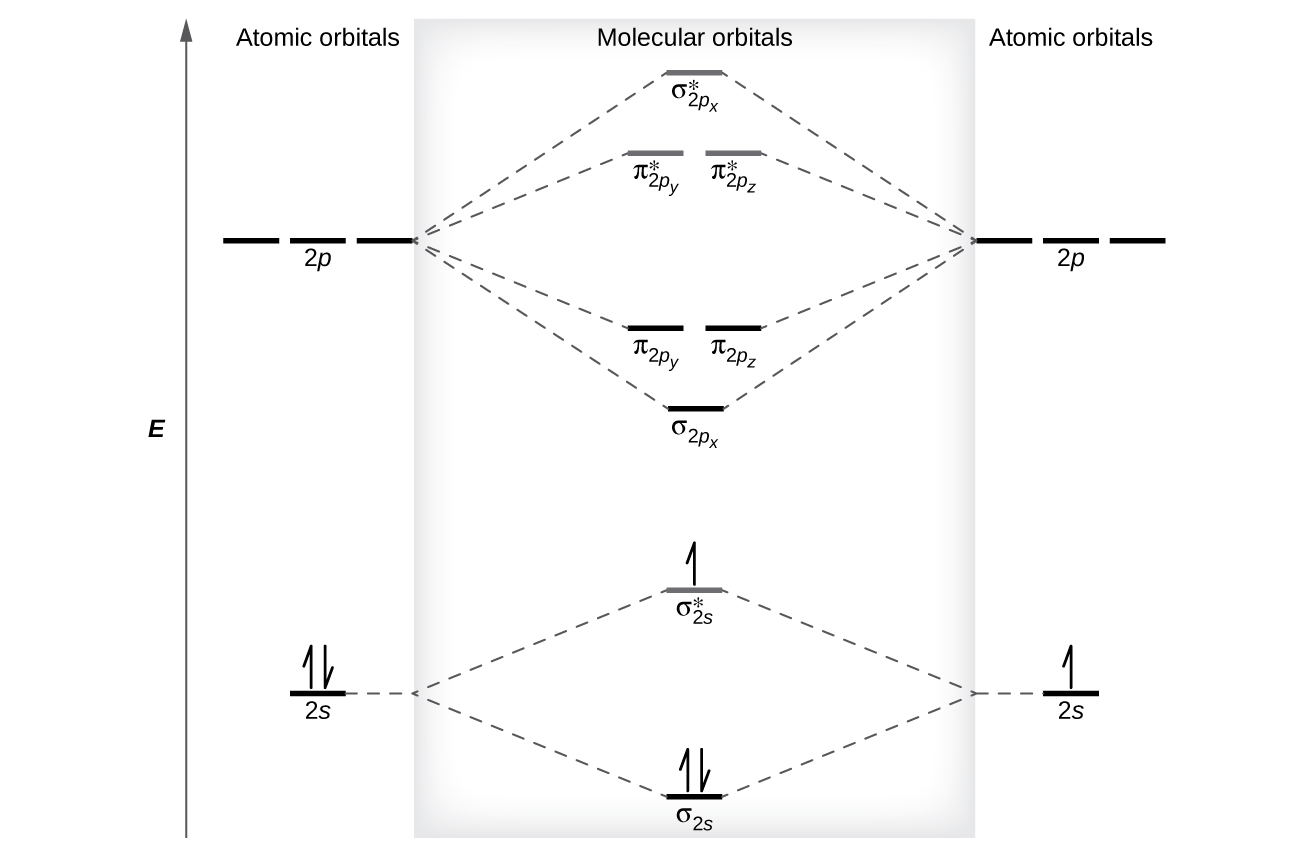
Molecular Orbital Theory Atoms First Openstax